
Animal feed is an essential part of the food chain and shows an enormous role in the safety and quality of animal by-products in the food supply chain. However, natural contamination of feed raw materials with fungal pathogens, before and after harvest, is a continuing and growing problem worldwide, as many of these fungal species produce mycotoxins that can profoundly affect animal health [1]. Zearalenone (ZEN), previously known as F-2 toxin, is a non-steroidal estrogenic mycotoxin that causes various changes and disorders related to the reproductive systems of most animal species, generating considerable economic losses in animal husbandry, especially for swine production [2-4]. ZEN and its metabolites pose a potential risk to mammals, especially when exposed to high doses over prolonged periods. Grain and balanced feeds from East Asia, Sub-Saharan Africa, and South America have the highest positive ZEN rate compared to other regions [5]. Maize is the most contaminated cereal, although ZEN has also been found in soybean, rice, rye, sorghum, oats, barley, and wheat products [6]. Zearalenone also often co-occurs with other Fusarium mycotoxins, mainly deoxynivalenol (DON) [7]. Both ZEN and DON can be produced by F. graminearum or F. culmorum, which means the most suitable moisture and temperature for Fusarium spp. growth and production of ZEN are the same that favor DON production [8]. Many global surveys indicate that the mycotoxin combinations of DON and ZEN are the most commonly detected in raw materials and finished feeds [5,9].
Zearalenone is a mycotoxin with strongly estrogenic and slightly hepatotoxic as well as immunotoxic effects [6,10,11]. The sensitivities of ZEN toxicities in farm animals depend on the metabolite distribution ratio of the animal and the situations of the reproductive system (e.g., male or female; adolescence or pregnancy stages) [12]. Prepubertal female piglets are more susceptible to ZEN exposure than mature sows [13]. Zearalenone is metabolized in the digestive system and has two primary metabolites: α-zearalenol (α-ZEL) and β-zearalenol (β-ZEL) [14,15]; they are formed via the reduction of ZEN. Zearalenone and its metabolites are structurally similar to endogenous estrogen hence, bind with estradiol receptors in the absence or low presence of estrogen resulting in morphological and biological alteration in reproductive organs [12]. Besides, ZEN cause reproductive changes due to higher estrogenic potential than other natural non-steroidal estrogenic compounds [16]. The α-ZEL has a greater affinity for estrogen receptors and is, therefore, more toxic than ZEN, while the β-ZEL has a lower affinity for these receptors, making it practically harmless [17]. The species of common economic animals most sensitive to the effects of ZEN is pigs, while the second most sensitive is ruminants, and the most resistant one is poultry [18]. The main reason for this phenomenon is that the main metabolite is α-ZEL in swine, while in ruminants and birds, it is β-ZEL [19,20]. The liver is the major organ of ZEN distribution, as ZEN induces histopathological changes and causes an increase in serum transaminases and bilirubin levels in animals at high concentrations in feed ingredients and animal feeds [6,21]. In general, ZEN does not negatively affect growth performances in farm animals unless it is present in extremely high levels in cereal and complete feeds [22]. Although ZEN is strongly estrogenic, fortunately, the amount of ZEN that carries over into final animal products (meat, eggs, and milk) is minimal under typical farming systems [2,23]; therefore, humans have less chance of indirect poisoning through the food chain system.
Table 1. The guidance values of the European Union (EU) Commission, United States Food and Drug Administration (FDA), and China for zearalenone concentrations (μg/kg) in complete feed [24-26].
| Animal Species | EU | FDA | China |
| Swine | - | ||
| Piglets and gilts | 100 | - | Gilts :100 ; Piglets : 150 |
| Sows and fattening pigs | 250 | - | 250 |
| Poultry | - | - | 500 |
| Ruminants | 500 | - | 500 |
Pigs are generally regarded as susceptible species to ZEN, the most sensitive being prepubertal female piglets, the second sensitive being mature sows, and the more tolerant being male swine compared to the former two [27,28]. The preliminary estrogenic effects of ZEN induce fertility disorders (infertility or reduced fertility) in swine [29]. Zearalenone inhibits the secretion of steroid hormones, interferes with the estrogen response in the pre-ovulatory phase, and inhibits mammal follicle maturation [30,31]. In female pigs, redness and swelling of the vulva, enlargement of the uterus and mammary glands, and cyst formation on the ovaries have been observed [32,33]. Zearalenone causes permanent estrus, pseudo-pregnancy, infertility, lower libido, stillbirths, and small litter in female swine upon extended exposure to higher concentrations of mycotoxin [12,34]. The European Commission stipulated limitations of 100 and 500 μg/kg for ZEN levels in the complete feed of piglets and gilts as well as sows and fattening pigs, respectively [25]. However, previous research reported that prepubertal female pigs fed 50 μg/kg of ZEN saw an increased number of vesicula follicles, while those fed 250 μg/kg of ZEN exhibited not only numerous vesicular follicles but also redness and swelling of the vulva, mammary glands swelling, and some cystic follicles on the ovaries [35]. On the other hand, ZEN induces feminization and testicular atrophy as well as reducing spermatogenesis, libido, and testosterone levels in male swine [32].
In addition to causing reproductive disorders, feeding female pigs 1.3 mg/kg of ZEN can reduce platelets, hemoglobin, globulin, triglycerides, and high-density lipoproteins in serum; increase enzyme activities; and lead to the degeneration of the liver and kidney [36]. Zearalenone is also a potential hepatotoxin and spleen toxin which induce oxidative stress and inflammation [37,38]. Besides, swine exposed to feed containing high doses of ZEN induced poor growth performances [39]. Previous meta-analysis research pointed out that feed containing an average of 3.8 mg/kg of ZEN resulted to 15.8% in feed intake and 28.8% in body weight gain reductions [40].
In terms of effects on future generations, in general, piglets can be exposed to ZEN in utero or by ingesting contaminated sow milk [38]. There are numerous cases of ZEN causing low reproductive performances in pigs in the field [13]. In Serbia and Germany, some swine farms observed such symptoms as increased rates of rebreeding, infertility, and anestrus in sows and a large number of stillborn and farrowed piglets with vulvovaginitis [13].
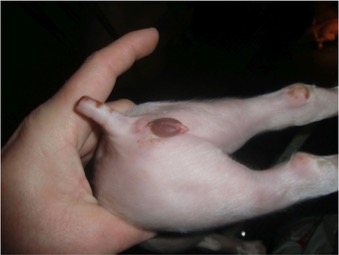
Fig. 1 Two-day old piglet of exposed sows with a swollen vulva. [13]
Table 1. Zearalenone (ZEN) toxicities on reproductive abilities and growth performances in swine
| Stage | ZEN level | Effects | Reference |
| Reproductive ability | |||
| Postweaning female piglets | >100 μg/kg | Inhibited follicles maturation and ovarian development; increased length, width, height, and area of vulval (vulvar malformation); reproductive related hormonal imbalance (e.g., luteinizing hormone) | [33,36,41] |
| prepubertal female piglets | >50 μg/kg | Increased vesicular follicles, redness and swelling of the vulva, mammary glands swelling, and some cystic follicles on the ovaries; reproductive related hormonal imbalance (e.g., luteinizing hormone) | [27,35,37] |
| Sow and gilts | >200 μg/kg | Disturbances of development and maturation of ovarian follicles; reproductive system disorders and abnormal estrous cycle; reproductive related hormonal imbalance (e.g., luteinizing hormone) | [42,43] |
| Pregnant sow | >250 μg/kg | Increased rates of rebreeding, infertility, and anestrus in sows and a large number of stillborn and farrowed piglets with vulvovaginitis | [44,45] |
| Mature male swine | >200 mg/kg | Feminization and testicular atrophy as well as reducing spermatogenesis, libido, and testosterone levels | [29,46] |
| Growth performance | |||
| Growing pig | >3 mg/kg | Decreased feed intake and body weight gain; damages of liver and spleen | [15,33,47] |
2.2 Poultry
Poultry responds to the presence of ZEN in the feed only at relatively high dietary concentrations and can generally be regarded as resistant [48]. There are two main conclusions regarding this phenomenon: The leading opinion to the naturally high concentration of estrogen in poultry blood, seeing as natural estrogens are considered to higher receptor affinity compare to ZEN [23]; the other opinion suggested that most research indicated ZEN was more extensively metabolized to less toxic α-ZEL than β-ZEL [49]. Zearalenone toxicities are observed only at exposure levels, hardly to occur under experimental poultry feeding conditions or continuous feeding for an exceedingly long period [48]. Furthermore, the EU has not imposed maximum ZEN limits because poultry has a high tolerance towards the substance as well [25]. Exposing adult laying hens to feed containing 0.75 mg/kg of ZEN for 35 days resulted in significant reduction in egg production compared with control group [3]. Besides, male turkeys fed 400 and 800 mg/kg of ZEN had a reduced sperm percentage and fertilization rate, and it promoted precocity [50]. Therefore, ZEN has low reproductive toxicity to poultry. Zearalenone almost did not affect growth performances (e.g., feed intake, body weight, and feed conversion ratio) unless chicks were fed a diet of over 2 mg/kg for 42 days [51]. In contrast, feeding ZEN-contaminated diets to turkeys leads to strutting behavior, increased size and coloration of caruncles and dewlaps, and swollen vent tissue [52]. An additional study indicated that purified ZEN increases oviduct development in growing female chickens and delays the growth of the testes in young male chickens [50]. In commercial feeding conditions, there are no detected ZEN residues in eggs from commercial production. However, previous research indicated that for feed containing high dose exposure of ZEN (10 mg/kg) in laying hens, significant levels of ZEN lipophilic metabolites might accumulate in the egg yolk if the exposure time was prolonged [53].
Table 2. Zearalenone (ZEN) toxicities on growth performances and reproductive abilities in poultry
| Stage | ZEN level | Effects | Reference |
| Laying hens | >0.4 mg/kg for more than seven weeks | Increased relative weight of oviduct and ovary, degeneration, and atrophy of the ovarian tissues | [54,55] |
| Laying hens | >0.75 mg/kg diet for more than 5 weeks | Increased feed conversion ratio (g feed/g egg), decreased egg production, albumen height, and Haugh unit | [3] |
| Broiler | > 2 mg/kg diet for more than 3 weeks | Decreased body weight gain, increased feed conversion ratio (g feed/g body weight), relative weight of liver, and liver damage | [4,51] |
2.3 Ruminants
The European Commission stipulated limitations of 500 μg/kg for ZEN levels in the complete feed of ruminants [25]. Zearalenone is produced in trace amounts in concentrates and forage (the average level of which being 25 to 100 μg/kg) occurring naturally [5], and rumen microbes extensively metabolize ZEN to generate less toxic metabolites (i.e., β-ZEL) [56]. Therefore, ruminants usually do not show symptoms of ZEN poisoning [2] unless highly superior levels of ZEN or other mycotoxins (e.g., DON) are both present in concentrates and forage [7]. Previous studies found that dairy herds receiving a diet contaminated with both DON and ZEN at levels of about 500 and 750 μg/kg, respectively, showed unsynchronized ovarian cycles, vaginitis, and early development of mammary glands in heifers [57]. However, heifers fed a diet containing only 1.25 mg/kg of ZEN did not show reproductive issues [58]. Zearalenone is toxic to ruminants at extremely high levels (at high doses rarely seen in nature) in naturally occurring concentrates and forages. Britain's research showed decreasing fertility in the dairy cows fed hay and grass silage containing ZEN at 14 mg/kg [59]. Another study indicated that dairy cows fed with ZEN-contaminated grain (25 mg/kg) show vaginitis, extended estrus, and decreased feed intake, and milk yield [59].
Currently, the only known field case is grazing sheep in New Zealand that were poisoned by ZEN, which caused infertility [60]. Most field or case reports in which a direct relationship between ZEN exposition levels and symptoms of estrogenic effects was not found were reported, suggesting this might reflect the variability in rumen degradation of ZEN. Previous research orally administered virgin heifers with an extremely high ZEN dose (250 mg) for 66 days, resulting in a slightly decreased conception rate (62%) compared to 87% found in the untreated control group) [48]. In general, ZEN and its metabolites can be detectable in the liver and bile, but most studies are not detected in milk because of their endogenous ruminal detoxification [61]. However, when dairy cows ingest exceptionally high levels of concentrates, that typically induces ZEN to carry over into milk. For instance, previous researchers found that 0.7% of ZEN could carry over into milk when 200 mg of ZEN/day is detected in the concentrate for 7 days [2].
Table 3. Zearalenone (ZEN) toxicities on growth performances and reproductive abilities in ruminants
| Animal/Stage | ZEN level | Duration | Effects | Reference |
| Beef heifer | 0.3 mg/kg | 98 day | Only decreased oocyte quality | [31] |
| Breeding cow | 1 mg/kg diet | 30 day | Increased ovarian antral follicle population; increased synthesis of AMH by granulosa cells; no effect of fertility | [62] |
| Dry cow | 5.9 mg/kg TMR | 2 day | Altered rumen microflora; decreased rumen pH; increased fiber breakdown; increased feed intake | [56] |
| Ewe | >3 mg/ewe/day | 10 day | Reproductive disorders, lower lambing percentages and infertility | [63] |
AMH: anti-müllerian hormone; TMR: total mixed ration.
1. Siri-Anusornsak, W.; Kolawole, O.; Mahakarnchanakul, W.; Greer, B.; Petchkongkaew, A.; Meneely, J.; Elliott, C.; Vangnai, K. The Occurrence and Co-Occurrence of Regulated, Emerging, and Masked Mycotoxins in Rice Bran and Maize from Southeast Asia. Toxins (Basel) 2022, 14, doi:10.3390/toxins14080567.
2. Liu, J.; Applegate, T. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins (Basel) 2020, 12, doi:10.3390/toxins12060377.
3. Yuan, T.; Li, J.; Wang, Y.; Li, M.; Yang, A.; Ren, C.; Qi, D.; Zhang, N. Effects of Zearalenone on Production Performance, Egg Quality, Ovarian Function and Gut Microbiota of Laying Hens. Toxins 2022, 14, 653.
4. Jia, S.; Ren, C.; Yang, P.; Qi, D. Effects of Intestinal Microorganisms on Metabolism and Toxicity Mitigation of Zearalenone in Broilers. Animals (Basel) 2022, 12, doi:10.3390/ani12151962.
5. Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins (Basel) 2019, 11, doi:10.3390/toxins11070375.
6. Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol 2007, 45, 1-18, doi:10.1016/j.fct.2006.07.030.
7. Thapa, A.; Horgan, K.A.; White, B.; Walls, D. Deoxynivalenol and Zearalenone-Synergistic or Antagonistic Agri-Food Chain Co-Contaminants? Toxins (Basel) 2021, 13, doi:10.3390/toxins13080561.
8. Kharbikar, L.L.; Dickin, E.T.; Edwards, S.G. Impact of post-anthesis rainfall, fungicide and harvesting time on the concentration of deoxynivalenol and zearalenone in wheat. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2015, 32, 2075-2085, doi:10.1080/19440049.2015.1084652.
9. DSM. DSM World Mycotoxin Survey; 2022.
10. Faisal, Z.; Garai, E.; Csepregi, R.; Bakos, K.; Fliszar-Nyul, E.; Szente, L.; Balazs, A.; Cserhati, M.; Koszegi, T.; Urbanyi, B., et al. Protective effects of beta-cyclodextrins vs. zearalenone-induced toxicity in HeLa cells and Tg(vtg1:mCherry) zebrafish embryos. Chemosphere 2020, 240, 124948, doi:10.1016/j.chemosphere.2019.124948.
11. Azam, M.S.; Yu, D.; Liu, N.; Wu, A. Degrading Ochratoxin A and Zearalenone Mycotoxins Using a Multifunctional Recombinant Enzyme. Toxins (Basel) 2019, 11, doi:10.3390/toxins11050301.
12. Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim Feed Sci Tech 2007, 137, 326-341, doi:https://doi.org/10.1016/j.anifeedsci.2007.06.008.
13. Hennig-Pauka, I.; Koch, F.J.; Schaumberger, S.; Woechtl, B.; Novak, J.; Sulyok, M.; Nagl, V. Current challenges in the diagnosis of zearalenone toxicosis as illustrated by a field case of hyperestrogenism in suckling piglets. Porcine Health Manag 2018, 4, 18, doi:10.1186/s40813-018-0095-4.
14. Olsen, M.; Pettersson, H.; Sandholm, K.; Visconti, A.; Kiessling, K.H. Metabolism of zearalenone by sow intestinal mucosa in vitro. Food Chem Toxicol 1987, 25, 681-683, doi:10.1016/0278-6915(87)90101-3.
15. Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet J 2006, 172, 96-102, doi:10.1016/j.tvjl.2005.03.004.
16. Bennett, J.W.; Klich, M. Mycotoxins. Clinical Microbiology Reviews 2003, 16, 497-516, doi:10.1128/CMR.16.3.497-516.2003.
17. Ropejko, K.; Twaruzek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins (Basel) 2021, 13, doi:10.3390/toxins13010035.
18. Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: a review. Food Production, Processing and Nutrition 2019, 1, 6, doi:10.1186/s43014-019-0007-2.
19. Mirocha, C.J.; Robison, T.S.; Pawlosky, R.J.; Allen, N.K. Distribution and residue determination of [3H]zearalenone in broilers. Toxicol Appl Pharmacol 1982, 66, 77-87, doi:10.1016/0041-008x(82)90062-x.
20. Buranatragool, K.; Poapolathep, S.; Isariyodom, S.; Imsilp, K.; Klangkaew, N.; Poapolathep, A. Dispositions and tissue residue of zearalenone and its metabolites alpha-zearalenol and beta-zearalenol in broilers. Toxicol Rep 2015, 2, 351-356, doi:10.1016/j.toxrep.2014.12.011.
21. Dänicke, S.; Winkler, J. Invited review: Diagnosis of zearalenone (ZEN) exposure of farm animals and transfer of its residues into edible tissues (carry over). Food and Chemical Toxicology 2015, 84, 225-249, doi:https://doi.org/10.1016/j.fct.2015.08.009.
22. Winkler, J.; Kersten, S.; Meyer, U.; Engelhardt, U.; Dänicke, S. Residues of zearalenone (ZEN), deoxynivalenol (DON) and their metabolites in plasma of dairy cows fed Fusarium contaminated maize and their relationships to performance parameters.
23. Völkel, I.; Schröer-Merker, E.; Czerny, C.-P. The Carry-Over of Mycotoxins in Products of Animal Origin with Special Regard to Its Implications for the European Food Safety Legislation. Food and Nutrition Sciences 2011, 02, 852-867, doi:10.4236/fns.2011.28117.
24. Park, D.L.; Troxell, T.C. U.S. perspective on mycotoxin regulatory issues.
25. Commission, E. Commission recommendation of of 17 august 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin a, T-2 and HT-2 and fumonisins in products intended for animal feeding. Commission, E., Ed. Official Journal of the European Union: 2006.
26. General Administration of Quality Supervision, I.a.Q.o.t.P.s.R.o.C.a.S.A.o.t.P.s.R.o.C. Hygienic Standard for Feeds GB13078-2017. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China and Standardization Administration of the People's Republic of China: 2018.
27. Xu, Y.; Sun, M.H.; Li, X.H.; Ju, J.Q.; Chen, L.Y.; Sun, Y.R.; Sun, S.C. Modified hydrated sodium calcium aluminosilicate-supplemented diet protects porcine oocyte quality from zearalenone toxicity. Environ Mol Mutagen 2021, 62, 124-132, doi:10.1002/em.22399.
28. Liu, C.; Chang, J.; Wang, P.; Yin, Q.; Huang, W.; Dang, X.; Lu, F.; Gao, T. Zearalenone Biodegradation by the Combination of Probiotics with Cell-Free Extracts of Aspergillus oryzae and its Mycotoxin-Alleviating Effect on Pig Production Performance. Toxins (Basel) 2019, 11, doi:10.3390/toxins11100552.
29. Tassis, P.D.; Reisinger, N.; Nagl, V.; Tzika, E.; Schatzmayr, D.; Mittas, N.; Basioura, A.; Michos, I.; Tsakmakidis, I.A. Comparative Effects of Deoxynivalenol, Zearalenone and Its Modified Forms De-Epoxy-Deoxynivalenol and Hydrolyzed Zearalenone on Boar Semen In Vitro. Toxins (Basel) 2022, 14, doi:10.3390/toxins14070497.
30. Zhang, G.L.; Feng, Y.L.; Song, J.L.; Zhou, X.S. Zearalenone: A Mycotoxin With Different Toxic Effect in Domestic and Laboratory Animals' Granulosa Cells. Front Genet 2018, 9, 667, doi:10.3389/fgene.2018.00667.
31. Silva, L.A.; de Mello, M.R.B.; Oliveira Pião, D.; Silenciato, L.N.; de Quadros, T.C.O.; de Souza, A.H.; Barbero, R.P. Effects of experimental exposure to zearalenone on reproductive system morphometry, plasma oestrogen levels, and oocyte quality of beef heifer. Reprod Domest Anim 2021, 56, 775-782, doi:10.1111/rda.13917.
32. Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of Zearalenone and Its Major Modified Forms in Pigs. LID - 10.3390/toxins9020056 [doi] LID - 56.
33. Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Gao, J.; Liu, F.X.; Broomhead, J.; Chi, F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J Anim Sci 2011, 89, 3008-3015, doi:10.2527/jas.2010-3658.
34. Shier, W.T.; Shier, A.C.; Xie, W.; Mirocha, C.J. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 2001, 39, 1435-1438, doi:10.1016/s0041-0101(00)00259-2.
35. Bauer, J.; Heinritzi, K.; Gareis, M.; Gedek, B. [Changes in the genital tract of female swine after feeding with practice-relevant amounts of zearalenone]. Tierarztl Prax 1987, 15, 33-36.
36. Jiang, S.; Yang, Z.; Yang, W.; Gao, J.; Liu, F.; Chen, C.-c.; Chi, F. Physiopathological effects of zearalenone in post-weaning female piglets with or without montmorillonite clay adsorbent. Livest Sci 2010, 131, 130-136, doi:https://doi.org/10.1016/j.livsci.2010.02.022.
37. Denli, M.; Blandon, J.C.; Guynot, M.E.; Salado, S.; Perez, J.F. Efficacy of activated diatomaceous clay in reducing the toxicity of zearalenone in rats and piglets. J Anim Sci 2015, 93, 637-645, doi:10.2527/jas.2014-7356.
38. Dänicke, S.; Brüssow, K.P.; Goyarts, T.; Valenta, H.; Ueberschär, K.H.; Tiemann, U. On the transfer of the Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) from the sow to the full-term piglet during the last third of gestation. Food Chem Toxicol 2007, 45, 1565-1574, doi:10.1016/j.fct.2007.02.016.
39. Wang, J.P.; Chi, F.; Kim, I.H. Effects of montmorillonite clay on growth performance, nutrient digestibility, vulva size, faecal microflora, and oxidative stress in weaning gilts challenged with zearalenone. Anim Feed Sci Tech 2012, 178, 158-166, doi:https://doi.org/10.1016/j.anifeedsci.2012.09.004.
40. Hee Mok, C.; Youp Shin, S.; Gyun Kim, B. Aflatoxin, deoxynivalenol, and zearalenone in swine diets: Predictions on growth performance. Revista Colombiana de Ciencias Pecuarias 2013, 26, 243-254.
41. Su, Y.; Sun, Y.; Ju, D.; Chang, S.; Shi, B.; Shan, A. The detoxification effect of vitamin C on zearalenone toxicity in piglets. Ecotoxicol Environ Saf 2018, 158, 284-292, doi:10.1016/j.ecoenv.2018.04.046.
42. Zwierzchowski, W.; Przybylowicz, M.; Obremski, K.; Zielonka, L.; Skorska-Wyszynska, E.; Gajecka, M.; Polak, M.; Jakimiuk, E.; Jana, B.; Rybarczyk, L., et al. Level of zearalenone in blood serum and lesions in ovarian follicles of sexually immature gilts in the course of zearalenone micotoxicosis. Pol J Vet Sci 2005, 8, 209-218.
43. Etienne, M.; Jemmali, M. Effects of zearalenone (F2) on estrous activity and reproduction in gilts. J Anim Sci 1982, 55, 1-10, doi:10.2527/jas1982.5511.
44. Zhou, J.; Zhao, L.; Huang, S.; Liu, Q.; Ao, X.; Lei, Y.; Ji, C.; Ma, Q. Zearalenone toxicosis on reproduction as estrogen receptor selective modulator and alleviation of zearalenone biodegradative agent in pregnant sows. J Anim Sci Biotechnol 2022, 13, 36, doi:10.1186/s40104-022-00686-3.
45. Shreeve, B.J.; Patterson, D.S.; Roberts, B.A.; Wrathall, A.E. Effect of mouldy feed containing zearalenone on pregnant sows. Br Vet J 1978, 134, 421-427, doi:10.1016/s0007-1935(17)33383-3.
46. Ruhr, L.P.; Osweiler, G.D.; Foley, C.W. Effect of the estrogenic mycotoxin zearalenone on reproductive potential in the boar. Am J Vet Res 1983, 44, 483-485.
47. Tiemann, U.; Brussow, K.P.; Kuchenmeister, U.; Jonas, L.; Pohland, R.; Reischauer, A.; Jager, K.; Danicke, S. Changes in the spleen and liver of pregnant sows and full-term piglets after feeding diets naturally contaminated with deoxynivalenol and zearalenone. Vet J 2008, 176, 188-196, doi:10.1016/j.tvjl.2007.02.019.
48. Chain, E.P.o.C.i.t.F.; Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L., et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J 2017, 15, e04851, doi:10.2903/j.efsa.2017.4851.
49. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 2014, 12, doi:10.2903/j.efsa.2014.3916.
50. Allen, N.K.; Mirocha, C.J.; Weaver, G.; Aakhus-Allen, S.; Bates, F. Effects of dietary zearalenone on finishing broiler chickens and young turkey poults. Poult Sci 1981, 60, 124-131, doi:10.3382/ps.0600124.
51. Chen, Y.; Cheng, Y.; Wen, C.; Wang, W.; Kang, Y.; Wang, A.; Zhou, Y. The protective effects of modified palygorskite on the broilers fed a purified zearalenone-contaminated diet. Poult Sci 2019, 98, 3802-3810, doi:10.3382/ps/pez085.
52. Olsen, M.; Mirocha, C.J.; Abbas, H.K.; Johansson, B. Metabolism of high concentrations of dietary zearalenone by young male turkey poults. Poult Sci 1986, 65, 1905-1910, doi:10.3382/ps.0651905.
53. Dailey, R.E.; Reese, R.E.; Brouwer, E.A. Metabolism of [14C]zearalenone in laying hens. J Agric Food Chem 1980, 28, 286-291, doi:10.1021/jf60228a008.
54. Cheng, Q.; Jiang, S.Z.; Li, S.Q.; Wang, Y.X.; Zhang, C.Y.; Yang, W.R. Effects of low-dose zearalenone-contaminated diets with or without montmorillonite clay adsorbent on nutrient metabolic rates, serum enzyme activities, and genital organs of growing-laying hens. J Appl Poultry Res 2017, 26, 367-375, doi:https://doi.org/10.3382/japr/pfx004.
55. Wu, Y.; Yang, W.; Yang, Z.; Jiang, S.; Zhang, G.; Jiang, X. Effects of low dose zearalenone and adsorbent on growth performance, serum biochemical and antioxidant indices of growing-laying hens. Chinese Journal of Animal Nutrition 2016, 28, 1137-1144.
56. Hartinger, T.; Grabher, L.; Pacifico, C.; Angelmayr, B.; Faas, J.; Zebeli, Q. Short-term exposure to the mycotoxins zearalenone or fumonisins affects rumen fermentation and microbiota, and health variables in cattle. Food Chem Toxicol 2022, 162, 112900, doi:10.1016/j.fct.2022.112900.
57. Coppock, R.W.; Mostrom, M.S.; Sparling, C.G.; Jacobsen, B.; Ross, S.C. Apparent zearalenone intoxication in a dairy herd from feeding spoiled acid-treated corn. Vet Hum Toxicol 1990, 32, 246-248.
58. Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins (Basel) 2015, 7, 3057-3111, doi:10.3390/toxins7083057.
59. Schuh, M. Clinical and subclinical events related to the presence of mycotoxins in cattle feed. The Bovine Practitioner 1998, 1998, 34-38, doi:10.21423/bovine-vol1998no32.1p34-38.
60. JF, S.; CA, M. Review of zearalenone studies with sheep in New Zealand. In Proceedings of Proceedings of the New Zealand Society of Animal Production, Napier, Jan; pp. 306-310.
61. Seeling, K.; Dänicke S Fau - Ueberschär, K.H.; Ueberschär Kh Fau - Lebzien, P.; Lebzien P Fau - Flachowsky, G.; Flachowsky, G. On the effects of Fusarium toxin-contaminated wheat and the feed intake level on the metabolism and carry over of zearalenone in dairy cows.
62. Fushimi, Y.; Takagi, M.; Monniaux, D.; Uno, S.; Kokushi, E.; Shinya, U.; Kawashima, C.; Otoi, T.; Deguchi, E.; Fink-Gremmels, J. Effects of Dietary Contamination by Zearalenone and Its Metabolites on Serum Anti-Mullerian Hormone: Impact on the Reproductive Performance of Breeding Cows. Reprod Domest Anim 2015, 50, 834-839, doi:10.1111/rda.12599.
63. Smith, J.F.; di Menna, M.E.; McGowan, L.T. Reproductive performance of Coopworth ewes following oral doses of zearalenone before and after mating. J Reprod Fertil 1990, 89, 99-106, doi:10.1530/jrf.0.0890099.
backNatural herb countaining feed additive for swine
Feed additive against the negative effects of mycotoxins for swine
Natural phytobiotic combination for poultry
A polysaccharidose enzyme containing feed supplement for ruminants
A probiotic innovation for the optimal microbiom
A natural phytobiotic, prebiotic combination for maintaining gut balance
Bioavailable minerals