Challenges During Weaning in Piglets: A Comprehensive Exploration of Pathoge

By: Dr. Ko-Hua Tso, scientific expert, Dr. Bata Ltd.
1. Introduction
Weaning is considered one of the most critical periods in pig management. It is associated with environmental, social, and dietary stress [1]. In modern intensive farming systems, early weaning techniques are often used to improve the productivity of sows, which can increase their annual litter size, improve the utilization rate of breeding equipment, and bring more economic benefits for breeding enterprises [2]. However, many stress factors associated with the weaning period, such as separation from the sow, dietary changes, adapting to a new environment, mixing of pigs from different farms, and histological changes in the small intestine, resulting in perturbation of the intestinal microbiota, intestinal digestion, absorption capacity, and mucosal immune function [3], which eventually leads to decreasing of feed intake, the occurrence of post-weaning diarrhea, growth reduction, even death [4]. Because the intestinal development of the piglets is not mature, the digestive system and immune system are not perfect at this stage, which leads to a poor ability to adapt to new environments when weaning piglets [4]. Simultaneously, due to the changes in digestive enzymes in the gastrointestinal tract, early-weaned piglets can’t digest solid feed well, destroying the intestinal physical barrier and unbalancing intestinal microbiota [5]. When piglets suffer weaning stress, the intestinal environment is susceptible to invasion by pathogenic microorganisms such as Escherichia coli (E. coli), which stimulates the intestinal mucosa to secrete inflammatory factors and damage the function of the intestinal mucosal barrier. Weaning stress is closely related to the immune system and intestinal barrier function [6]. Furthermore, weaning stress causes gut microbiota dysbiosis and increases piglets' risk of gastrointestinal diseases [7]. All pig producers should correctly understand the effects of weaning stress and pathogenic infections in piglets.
2. Weanling Challenge
Weaning is a significant stressor in the life of a piglet. It refers to transitioning piglets from sow milk to solid feed and water [8], usually occurring between 3 and 6 weeks of age [9]. This period can be highly stressful for piglets.
- Separation from the Sow [10]: Piglets are naturally attached to their sows. Weaning involves separating piglets from their sows and littermates, leading to emotional stress as they lose their source of comfort and security.
- Dietary Changes: Weaning introduces significant dietary changes. The piglets must cope with the sudden withdrawal of sow milk and adapt to less-digestible, plant-based dry diets containing complex protein and carbohydrates, including various anti-nutritional factors [11]. Piglets need to adapt to consuming solid feed and drinking water, which can be challenging for their developing digestive systems. This change in diet can lead to gastrointestinal disturbances and nutritional stress [12]. Therefore, many piglets have a sharp reduction in feed intake immediately after weaning [13]. Approximately 50% of weaned pigs consume their first feed within 24 hours post-weaning, and in about 10% of weaning piglets, anorexia persists for up to 48 hours [14]. Inadequate feed intake after weaning results in insufficient dietary nutrient utilization and local inflammation [15]. Regarding more details of gastrointestinal changes are listed in part 3.
- Social Stress [16]: Weaning often involves moving piglets to new pens or locations. New social structures form, and piglets have to establish new hierarchies within their groups. Social stress can arise due to competition for resources, space, and social dominance.
- Environmental Changes [17]: Weaned piglets may be moved to different environments, which can include changes in temperature, lighting, and overall housing conditions. These changes can be stressful, especially if the new environment needs to be managed correctly.
- Pathogen Exposure [1]: Weaning can increase disease susceptibility. Stress compromises the piglet's immune system, making them more vulnerable to infections. Exposure to new pathogens in the new environment can lead to disease outbreaks. More details are listed in part 4.
- Transportation Stress [18]: The journey can be highly stressful if piglets are transported during the weaning process. Handling, loading, and unloading procedures and the noise and movement during transportation can cause significant stress.
- Handling Stress [19]: Weaned piglets are often handled more frequently for health checks, vaccinations, and other management practices. Handling can be stressful, especially if not done gently and with care.
3. Gastrointestinal changes
As the leading site of nutrient digestion and absorption and a vital defense line against the invasion of bacteria and endotoxins into the intestinal lumen [20], the intestinal tract is an important organ in response to piglet stress. Therefore, it is important to maintain intestinal health in pig production. Even though weaning ages at large commercial farms have been increased to 3 to 4 weeks, pigs are naturally weaned at the age of 12 to 17 weeks [21]. Early weaning causes morphological changes in the small intestine of pigs [22]. For instance, the small intestinal villus height and crypt depth of 21-day-old piglets decreased and increased during day 11 post-weaning. Due to morphological changes, intestinal functionality also changes. To accommodate the transition from milk to solid feed, the main digestive enzyme changes in the gastrointestinal are that lactase decreases while amylase, maltase, lipase, pepsin, and trypsin increase [23,24]. In the meantime, more nutrients are being digested and absorbed in the intestine [25].
On the other hand, the relationship between pigs' intestinal microbiota and growth performance is of great importance for improving piglet's health [8]. Various studies found that gut microbiota variation is significantly associated with porcine body weight gain (BWG) during weaning and the early nursery period [26,27]. Numerous studies have shown that weaning transition reduced the abundance of Lactobacillus and gut microbiota diversity, whereas Clostridium and E. coli significantly increased, inducing post-weaning diarrhea [28,29]. Previous research [28] indicated that the succession of the gut microbiota in high-BWG piglets occurred faster and stabilized sooner upon weaning. Hence, maintaining the balance and abundance of porcine gastrointestinal microbiota plays an essential role in pig production, especially in the piglet stage.
4. Pathogen bacterial infection
The weaning period is marred by challenges, especially concerning pathogenic bacterial infections, such as Escherichia coli, Brachyspira hyodysenteriae, Lawsonia intracellularis, Clostridium perfringens, and Salmonella [30-32]. Post-weaning diarrhea mainly affects piglets during the first 2 weeks after weaning and is characterized by sudden death or diarrhea, dehydration, and growth retardation in surviving piglets, causing heavy economic losses in pig production worldwide [33]. It should be noted that almost 49% of neonatal and young piglet deaths are caused by diarrhea [34].
4. 1 Escherichia coli
Colibacillosis is a common disease in suckling and weaning pigs [35]. At birth, E. coli colonization of the gut microbiota begins once the newborn is exposed to microbes from the sows and the surrounding environment and is influenced by the consumption of colostrum and milk in sows [36]. Subsequently, aerobic bacteria, facultative anaerobes, and obligate anaerobes gradually colonized the intestinal tract of piglets, and about 2 days later, microorganisms wholly colonized the intestinal tract, and Lactobacillus was the dominant bacteria [37]. In early weaning, the diet of piglets is changed from good digestible sow milk to poor digestible solid feed, and piglets can’t fully digest and utilize these nutrients due to the immature digestive system, which provides a good source of nutrients for some pathogenic bacteria to multiply, thereby altering the composition of the gut microbiota (e.g., E. coli increasing and Lactobacillus decreasing) and destroying the microbial barrier function of the intestinal tract [38,39]. These strains of E. coli have fimbriae or pili that allow them to adhere to the jejunum and ileum epithelium and produce enterotoxins [40]. Pathogenic strains produce enterotoxins that cause fluids and electrolytes to be secreted into the intestinal lumen, resulting in diarrhea [41]. The prevalence of E. coli in pig farms is concerning, making it a significant focus of research and intervention strategies. In some Asian countries (e.g., Japan), the proportion of this pathogen is 35% [42]. Symptoms of this bacterial are listed as follows:
- Diarrhea [33,43]: Once piglets are weaned, E. coli has a high frequency of infecting them. This category shows signs such as lethargy and disorientation with a noticeable drop in feed intake. Feces can be whitish, yellowish, or brown. Diarrhea can be persistent, leading to weight loss and poor growth rates. Sometimes, the diarrhea comes with lethargy and disorientation.
- Dehydration and Lethargy [33]: Diarrhea caused by E. coli infections can result in dehydration, which can be life-threatening, especially in young piglets. Dehydrated piglets may appear lethargic and weak and have sunken eyes.
- Loss of Appetite [44]: Infected piglets may lose their appetite, leading to reduced feed intake, further exacerbating body weight loss and slow growth.
- Respiratory Distress [45]: In severe cases, E. coli infection can lead to respiratory distress, characterized by labored breathing and coughing.
- Edema [46]: In cases of edema disease caused by specific strains of E. coli, piglets may develop swelling, particularly around the eyes, face, and neck.
- Sudden Death [47]: In some instances, particularly with septicemia, piglets may die suddenly without exhibiting many visible symptoms.
4.2 Brachyspira hyodysenteriae
B. hyodysenteriae, the causative agent of swine dysentery (SD), is prevalent in intensive pig farming. It induces mucohemorrhagic diarrhea, resulting in poor weight gain and economic losses for farmers [48]. In farrow-to-finish herds (including farrow-to-weaners and farrow-to-growers piggeries), the pathogen can persist in endemic infected sows, which have overcome the infection and developed protective immunity but still shed the pathogen in their feces. The bacterium can survive in the environment for extended periods and spreads through direct pig-to-pig contact or via contaminated feces, feed, water, or equipment [49]. Therefore, the proximity of facilities and the continuous flow of pigs in this sort of production system will facilitate the transmission of infection to non-infected piglets. Symptoms of this bacterial are listed as follows:
- Diarrhea [50]: The hallmark of SD is severe mucohemorrhagic diarrhea. The feces are often watery, bloody, and foul-smelling. This symptom is usually accompanied by straining during defecation.
- Dehydration and Lethargy [51]: Similar to the symptoms of E. coli. Prolonged diarrhea can lead to dehydration, which can be severe and life-threatening, especially in young piglets. Dehydrated piglets may appear weak and lethargic and have sunken eyes.
- Anorexia [48]: Infected piglets may lose their appetite, leading to reduced feed intake and subsequent weight loss.
- Weight Loss [50]: Diarrhea, decreased appetite, and dehydration can cause significant weight loss in affected piglets.
4.3 Lawsonia intracellularis
L. intracellularis causes porcine proliferative enteropathy (PPE) in piglets, leading to weight loss and decreased feed efficiency [52]. Infected pigs shed the bacterium in their feces, and transmission occurs through the oral-fecal route [53]. Like B. hyodysenteriae, carrier pigs can shed L. intracellularis without displaying clinical signs. Carrier sows can pass the bacterium to their piglets, contributing to the spread of the disease within a herd [54]. Symptoms of this bacterial are listed as follows:
- Diarrhea [52]: Infected piglets often develop watery to mucoid diarrhea. The severity of diarrhea can vary, but it often leads to dehydration.
- Weight Loss [55]: Diarrhea and decreased appetite result in weight loss and poor growth rates. Affected piglets fail to thrive and may become emaciated.
- Dehydration and Lethargy [56]: Persistent diarrhea leads to dehydration, which can be severe and life-threatening if not addressed promptly. Infected piglets may appear weak, lethargic, and less active than healthy piglets.
- Variable Appetite [57]: Piglets may have a decreased appetite, leading to reduced feed intake and subsequent weight loss.
4.4 Clostridium perfringens
C. perfringens is responsible for enteric diseases and causes sudden death and enteritis in piglets [58]. Its impact on mortality rates and economic losses is substantial. Symptoms of this bacterial are listed as follows:
- Diarrhea [59]: C. perfringens infections often cause watery to mucoid diarrhea in piglets. Diarrhea can be severe and may contain blood in some cases.
- Dehydration: [60]: Prolonged diarrhea can lead to dehydration, which can be life-threatening, especially in young piglets. Dehydrated piglets may appear weak, lethargic, and have sunken eyes.
- Abdominal Pain [32]: Piglets with C. perfringens infections may exhibit signs of abdominal discomfort, such as hunching, stretching, or vocalization, indicating pain.
- Loss of Appetite [61]: Infected piglets may lose their appetite, leading to reduced feed intake and poor growth rates.
- Weight Loss [62]: Diarrhea and decreased appetite can cause significant weight loss in affected piglets.
- Septic Shock [63]: In severe cases, the toxins produced by C. perfringens can cause toxic shock, leading to rapid deterioration of health and, in some cases, sudden death.
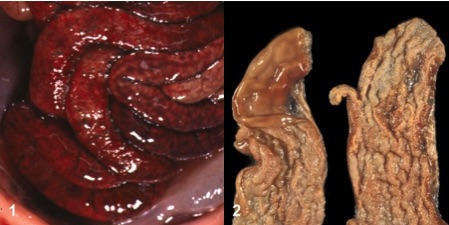
Figure 1. (1) Acute necrotic (serosal view) (2) Sub-acute (mucosal view) enteritis by C. perfringens type C in a piglet [32].
4.5 Salmonella
Salmonella destroys the intestinal mucosal mechanical barrier by inducing apoptosis of intestinal epithelial cells and disrupting the distribution of tight junction proteins between intestinal mucosal cells, causing enteritis, septicemia, and increased mortality rates [64]. The prevalence of Salmonella poses a significant challenge to swine farms globally. Symptoms of this bacterial are listed as follows
- Diarrhea [65]: Salmonella infections often cause watery mucoid diarrhea in piglets. Diarrhea can be severe and persistent, leading to dehydration.
- Dehydration and Lethargy [66]: Like the previous pathogenic bacteria, prolonged diarrhea can result in dehydration, which can be life-threatening, especially in young piglets. Dehydrated piglets may appear weak, lethargic, and have sunken eyes.
- Fever [66]: Infected piglets may have an elevated body temperature, indicating the presence of an infection.
- Loss of Appetite [67]: Piglets with Salmonella infections may lose their appetite, leading to reduced feed intake and poor growth rates.
- Weight Loss [68]: Diarrhea and decreased appetite can cause significant weight loss in affected piglets.
- Septicemia [65]: Salmonella infections can lead to septicemia, where bacteria spread throughout the bloodstream, causing systemic illness and sudden death in piglets.

Figure 2. Purple skin discoloration, pneumonia and rapid death associated with host-adapted systemic infection with Salmonella Choleraesuis.
(https://porkgateway.org/resource/salmonellosis-and-salmonella-infections/)
Conclusion
Weaning is considered one of the most critical periods in pig production, which is related to the economic benefits of pig farms. Farmers should use various management practices to minimize stress during the weaning period. Creating a nurturing environment through comfortable housing, quality nutrition, and gradual weaning techniques is fundamental. Furthermore, addressing the specific challenges posed by pathogens like E. coli, B. hyodysenteriae, L. intracellularis, C. perfringens, and Salmonella is paramount. By understanding their nuances and employing targeted interventions, the swine industry can ensure the smooth transition of piglets to independent feeding. These strategic solutions not only enhance the well-being of weaned piglets but also lay the foundation for healthier, faster-growing pigs and support the reproductive health of sows. Integrating rigorous biosecurity protocols, comprehensive vaccination strategies, and responsible feed additives (such as probiotics, prebiotics, or phytobiotics) represents a crucial step forward [69]. This multifaceted approach not only mitigates the impact of pathogens but also paves the way for a resilient and prosperous era in the swine industry, fostering sustainability and productivity in pig farming operations. Hence, the following articles will introduce more details of these target pathogens from multiple aspects such as description, prevalence, symptoms, and treatments. Furthermore, sow and fattening pig management are crucial components of pig production management as well. Effective management of both sows and fattening pigs is essential for the overall success, sustainability, and profitability of pig farming operations. Therefore, we will also arrange the following articles to discuss the challenges of sows and fattening pigs.
References
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front Immunol 2022, 13, 1042778, doi:10.3389/fimmu.2022.1042778.
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J Anim Sci Biotechnol 2013, 4, 19, doi:10.1186/2049-1891-4-19.
- Yu, L.; Li, H.; Peng, Z.; Ge, Y.; Liu, J.; Wang, T.; Wang, H.; Dong, L. Early Weaning Affects Liver Antioxidant Function in Piglets. Animals (Basel) 2021, 11, doi:10.3390/ani11092679.
- Su, W.; Gong, T.; Jiang, Z.; Lu, Z.; Wang, Y. The Role of Probiotics in Alleviating Postweaning Diarrhea in Piglets From the Perspective of Intestinal Barriers. Front Cell Infect Microbiol 2022, 12, 883107, doi:10.3389/fcimb.2022.883107.
- Ma, X.; Zhang, Y.; Xu, T.; Qian, M.; Yang, Z.; Zhan, X.; Han, X. Early-Life Intervention Using Exogenous Fecal Microbiota Alleviates Gut Injury and Reduce Inflammation Caused by Weaning Stress in Piglets. Front Microbiol 2021, 12, 671683, doi:10.3389/fmicb.2021.671683.
- Corino, C.; Prost, M.; Pizzi, B.; Rossi, R. Dietary Plant Extracts Improve the Antioxidant Reserves in Weaned Piglets. Antioxidants (Basel) 2021, 10, doi:10.3390/antiox10050702.
- Gresse, R.; Chaucheyras-Durand, F.; Denis, S.; Beaumont, M.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Weaning-associated feed deprivation stress causes microbiota disruptions in a novel mucin-containing in vitro model of the piglet colon (MPigut-IVM). J Anim Sci Biotechnol 2021, 12, 75, doi:10.1186/s40104-021-00584-0.
- Luo, Y.; Ren, W.; Smidt, H.; Wright, A.G.; Yu, B.; Schyns, G.; McCormack, U.M.; Cowieson, A.J.; Yu, J.; He, J., et al. Dynamic Distribution of Gut Microbiota in Pigs at Different Growth Stages: Composition and Contribution. Microbiol Spectr 2022, 10, e0068821, doi:10.1128/spectrum.00688-21.
- Holman, D.B.; Gzyl, K.E.; Mou, K.T.; Allen, H.K. Weaning Age and Its Effect on the Development of the Swine Gut Microbiome and Resistome. mSystems 2021, 6, e0068221, doi:10.1128/mSystems.00682-21.
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci 2013, 91, 1094-1101, doi:10.2527/jas.2012-5796.
- Lallès, J.-P.; Boudry, G.; Favier, C.; Floc’h, N.L.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: physiology. Anim. Res. 2004, 53, 301-316.
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int J Mol Sci 2022, 23, doi:10.3390/ijms23179588.
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livestock Production Science 1997, 51, 215-236, doi:https://doi.org/10.1016/S0301-6226(97)00057-2.
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr (Berl) 2013, 97, 207-237, doi:10.1111/j.1439-0396.2012.01284.x.
- McCracken, B.A.; Spurlock, M.E.; Roos, M.A.; Zuckermann, F.A.; Gaskins, H.R. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J Nutr 1999, 129, 613-619, doi:10.1093/jn/129.3.613.
- Clouard, C.; Resmond, R.; Vesque-Annear, H.; Prunier, A.; Merlot, E. Pre-weaning social behaviours and peripheral serotonin levels are associated with behavioural and physiological responses to weaning and social mixing in pigs. Applied Animal Behaviour Science 2023, 259, 105833, doi:https://doi.org/10.1016/j.applanim.2023.105833.
- Ramirez, B.C.; Hayes, M.D.; Condotta, I.; Leonard, S.M. Impact of housing environment and management on pre-/post-weaning piglet productivity. J Anim Sci 2022, 100, doi:10.1093/jas/skac142.
- Roldan-Santiago, P.; Trujillo-Ortega, M.; Borderas-Tordesillas, F.; Martinez-Rodriguez, R.; Mora-Medina, P.; Flores-Peinado, S.; Sanchez-Hernandez, M.; Garcia-Herrera, R.; Gonzalez-Lozano, M.; Mota-Rojas, D. Physiometabolic responses to road transport in weaned piglets for a short period and the effects of straw bedding. Anim Sci J 2015, 86, 563-571, doi:10.1111/asj.12324.
- Wang, C.; Chen, Y.; Bi, Y.; Zhao, P.; Sun, H.; Li, J.; Liu, H.; Zhang, R.; Li, X.; Bao, J. Effects of Long-Term Gentle Handling on Behavioral Responses, Production Performance, and Meat Quality of Pigs. Animals (Basel) 2020, 10, doi:10.3390/ani10020330.
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential Application of Lonicera japonica Extracts in Animal Production: From the Perspective of Intestinal Health. Front Microbiol 2021, 12, 719877, doi:10.3389/fmicb.2021.719877.
- Jensen, P. Observations on the maternal behaviour of free-ranging domestic pigs. Applied Animal Behaviour Science 1986, 16, 131-142, doi:https://doi.org/10.1016/0168-1591(86)90105-X.
- Montagne, L.; Boudry, G.; Favier, C.; Le Huerou-Luron, I.; Lalles, J.P.; Seve, B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br J Nutr 2007, 97, 45-57, doi:10.1017/S000711450720580X.
- Christensen, B.; Huber, L.A. The effects of creep feed composition and form and nursery diet complexity on small intestinal morphology and jejunal mucosa-specific enzyme activities after weaning in pigs. J Anim Sci 2022, 100, doi:10.1093/jas/skac138.
- Hedemann, M.S.; Jensen, B.B. Variations in enzyme activity in stomach and pancreatic tissue and digesta in piglets around weaning. Arch Anim Nutr 2004, 58, 47-59, doi:10.1080/00039420310001656677.
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim Sci J 2020, 91, e13357, doi:10.1111/asj.13357.
- Mahmud, M.R.; Jian, C.; Uddin, M.K.; Huhtinen, M.; Salonen, A.; Peltoniemi, O.; Venhoranta, H.; Oliviero, C. Impact of Intestinal Microbiota on Growth Performance of Suckling and Weaned Piglets. Microbiol Spectr 2023, 11, e0374422, doi:10.1128/spectrum.03744-22.
- Cremonesi, P.; Biscarini, F.; Castiglioni, B.; Sgoifo, C.A.; Compiani, R.; Moroni, P. Gut microbiome modifications over time when removing in-feed antibiotics from the prophylaxis of post-weaning diarrhea in piglets. PloS one 2022, 17, e0262199.
- Gaukroger, C.H.; Stewart, C.J.; Edwards, S.A.; Walshaw, J.; Adams, I.P.; Kyriazakis, I. Changes in Faecal Microbiota Profiles Associated With Performance and Birthweight of Piglets. Front Microbiol 2020, 11, 917, doi:10.3389/fmicb.2020.00917.
- Dou, S.; Gadonna-Widehem, P.; Rome, V.; Hamoudi, D.; Rhazi, L.; Lakhal, L.; Larcher, T.; Bahi-Jaber, N.; Pinon-Quintana, A.; Guyonvarch, A., et al. Characterisation of Early-Life Fecal Microbiota in Susceptible and Healthy Pigs to Post-Weaning Diarrhoea. PLoS One 2017, 12, e0169851, doi:10.1371/journal.pone.0169851.
- Plawinska, J.; Jakubowski, T.; Rzewuska, M.; Binek, M. Occurrence of Lawsonia Intracellularis and Brachyspira spp. infection in swine suffering from diarrhoea. Pol J Vet Sci 2004, 7, 203-206.
- Wang, Y.; Yan, X.; Zhang, W.; Liu, Y.; Han, D.; Teng, K.; Ma, Y. Lactobacillus casei Zhang Prevents Jejunal Epithelial Damage to Early-Weaned Piglets Induced by Escherichia coli K88 via Regulation of Intestinal Mucosal Integrity, Tight Junction Proteins and Immune Factor Expression. J Microbiol Biotechnol 2019, 29, 863-876, doi:10.4014/jmb.1903.03054.
- Uzal, F.A.; Navarro, M.A.; Asin, J.; Boix, O.; Ballarà-Rodriguez, I.; Gibert, X. Clostridial diarrheas in piglets: A review. Veterinary Microbiology 2023, 280, 109691, doi:https://doi.org/10.1016/j.vetmic.2023.109691.
- Fairbrother, J.M.; Nadeau, E.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev 2005, 6, 17-39, doi:10.1079/ahr2005105.
- Zheng, X.; Nie, K.; Xu, Y.; Zhang, H.; Xie, F.; Xu, L.; Zhang, Z.; Ding, Y.; Yin, Z.; Zhang, X. Fecal Microbial Structure and Metabolic Profile in Post-Weaning Diarrheic Piglets. Genes (Basel) 2023, 14, doi:10.3390/genes14061166.
- Barros, M.M.; Castro, J.; Araujo, D.; Campos, A.M.; Oliveira, R.; Silva, S.; Outor-Monteiro, D.; Almeida, C. Swine Colibacillosis: Global Epidemiologic and Antimicrobial Scenario. Antibiotics (Basel) 2023, 12, doi:10.3390/antibiotics12040682.
- Looft, T.; Allen, H.K.; Cantarel, B.L.; Levine, U.Y.; Bayles, D.O.; Alt, D.P.; Henrissat, B.; Stanton, T.B. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. The ISME Journal 2014, 8, 1566-1576, doi:10.1038/ismej.2014.12.
- Beaumont, M.; Cauquil, L.; Bertide, A.; Ahn, I.; Barilly, C.; Gil, L.; Canlet, C.; Zemb, O.; Pascal, G.; Samson, A., et al. Gut Microbiota-Derived Metabolite Signature in Suckling and Weaned Piglets. J Proteome Res 2021, 20, 982-994, doi:10.1021/acs.jproteome.0c00745.
- Shin, D.; Chang, S.Y.; Bogere, P.A.-O.; Won, K.; Choi, J.Y.; Choi, Y.J.; Lee, H.K.; Hur, J.; Park, B.Y.; Kim, Y., et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs.
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol 2017, 25, 851-873, doi:10.1016/j.tim.2017.05.004.
- Torres, A.G.; Zhou, X.; Kaper, J.B. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect Immun 2005, 73, 18-29, doi:10.1128/IAI.73.1.18-29.2005.
- Zhang, Y.; Tan, P.; Zhao, Y.; Ma, X. Enterotoxigenic Escherichia coli: intestinal pathogenesis mechanisms and colonization resistance by gut microbiota. Gut Microbes 2022, 14, 2055943, doi:10.1080/19490976.2022.2055943.
- Harada, K.; Asai, T.; Kojima, A.; Oda, C.; Ishihara, K.; Takahashi, T. Antimicrobial susceptibility of pathogenic Escherichia coli isolated from sick cattle and pigs in Japan. J Vet Med Sci 2005, 67, 999-1003, doi:10.1292/jvms.67.999.
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: risk factors and non-colistin-based control strategies. Acta Vet Scand 2017, 59, 31, doi:10.1186/s13028-017-0299-7.
- Kim, K.; Song, M.; Liu, Y.; Ji, P. Enterotoxigenic Escherichia coli infection of weaned pigs: Intestinal challenges and nutritional intervention to enhance disease resistance. Front Immunol 2022, 13, 885253, doi:10.3389/fimmu.2022.885253.
- Nakamine, M.; Kono Y Fau - Abe, S.; Abe S Fau - Hoshino, C.; Hoshino C Fau - Shirai, J.; Shirai J Fau - Ezaki, T.; Ezaki, T. Dual infection with enterotoxigenic Escherichia coli and porcine reproductive and respiratory syndrome virus observed in weaning pigs that died suddenly.
- Frydendahl, K. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet Microbiol 2002, 85, 169-182, doi:10.1016/s0378-1135(01)00504-1.
- 4Amezcua, R.; Friendship Rm Fau - Dewey, C.E.; Dewey Ce Fau - Gyles, C.; Gyles C Fau - Fairbrother, J.M.; Fairbrother, J.M. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns.
- 4Alvarez-Ordonez, A.; Martinez-Lobo, F.J.; Arguello, H.; Carvajal, A.; Rubio, P. Swine dysentery: aetiology, pathogenicity, determinants of transmission and the fight against the disease. Int J Environ Res Public Health 2013, 10, 1927-1947, doi:10.3390/ijerph10051927.
- Songer, J.G.; Harris, D.L. Transmission of swine dysentery by carrier pigs. Am J Vet Res 1978, 39, 913-916.
- Wills, R.W. Diarrhea in growing-finishing swine. Vet Clin North Am Food Anim Pract 2000, 16, 135-161, doi:10.1016/s0749-0720(15)30140-7.
- Nielsen Ss Fau - Bicout, D.J.; Bicout Dj Fau - Calistri, P.; Calistri P Fau - Canali, E.; Canali E Fau - Drewe, J.A.; Drewe Ja Fau - Garin-Bastuji, B.; Garin-Bastuji B Fau - Gonzales Rojas, J.L.; Gonzales Rojas Jl Fau - Gortázar, C.; Gortázar C Fau - Herskin, M.; Herskin M Fau - Michel, V.; Michel V Fau - Miranda Chueca, M.Á., et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): antimicrobial-resistant Brachyspira hyodysenteriae in swine.
- Karuppannan, A.K.; Opriessnig, T. Lawsonia intracellularis: Revisiting the Disease Ecology and Control of This Fastidious Pathogen in Pigs. Front Vet Sci 2018, 5, 181, doi:10.3389/fvets.2018.00181.
- McOrist, S.; Jasni, S.; Mackie, R.A.; MacIntyre, N.; Neef, N.; Lawson, G.H. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect Immun 1993, 61, 4286-4292, doi:10.1128/iai.61.10.4286-4292.1993.
- Karuppannan, A.K.; Opriessnig, T. Lawsonia intracellularis: Revisiting the Disease Ecology and Control of This Fastidious Pathogen in Pigs. Frontiers in Veterinary Science 2018, 5, doi:10.3389/fvets.2018.00181.
- Campillo, M.; Smith, S.H.; Gally, D.L.; Opriessnig, T. Review of methods for the detection of Lawsonia intracellularis infection in pigs. J Vet Diagn Invest 2021, 33, 621-631, doi:10.1177/10406387211003551.
- Peixoto, P.V.; França, T.N.; Ribeiro, C.T.; Bezerra Jr, P.S.; Driemeier, D. Proliferative enteropathy (Lawsonia intracellularis) outbreak in rabbits in Brazil. Pesquisa Veterinária Brasileira 2008, 28.
- Visscher, C.; Mischok, J.; Sander, S.; Schmicke, M.; Peitzmeier, E.U.; von dem Busche, I.; Rohn, K.; Kamphues, J. Nutrient digestibility, organ morphometry and performance in vaccinated or non-vaccinated Lawsonia intracellularis infected piglets. BMC Vet Res 2018, 14, 323, doi:10.1186/s12917-018-1662-2.
- Aldape, M.J.; Bryant, A.E.; Stevens, D.L. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin Infect Dis 2006, 43, 1436-1446, doi:10.1086/508866.
- Songer, J.G.; Uzal, F.A. Clostridial enteric infections in pigs. J Vet Diagn Invest 2005, 17, 528-536, doi:10.1177/104063870501700602.
- Diab, S.S. Diseases Produced by Clostridium perfringens Type C. In Clostridial Diseases of Animals, 2016; https://doi.org/10.1002/9781118728291.ch12pp. 143-155.
- Robbins, R.C.; Almond, G.; Byers, E. Swine Diseases and Disorders.
- Yaeger, M.J.; Kinyon, J.M.; Songer, J.G. A Prospective, Case Control Study Evaluating the Association between Clostridium Difficile Toxins in the Colon of Neonatal Swine and Gross and Microscopic Lesions. Journal of Veterinary Diagnostic Investigation 2007, 19, 52-59, doi:10.1177/104063870701900108.
- Fernandez, R.; Anampa-Guzmán, A. Septic Shock Due to Clostridium perfringens.
- Shu, L.Z.; Ding, Y.D.; Xue, Q.M.; Cai, W.; Deng, H. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Therap Adv Gastroenterol 2023, 16, 17562848231176427, doi:10.1177/17562848231176427.
- Dors, A.; Pomorska-Mol, M.; Czyzewska, E.; Wasyl, D.; Pejsak, Z. Prevalence and risk factors for Lawsonia intracellularis, Brachyspira hyodysenteriae and Salmonella spp. in finishing pigs in Polish farrow-to-finish swine herds. Pol J Vet Sci 2015, 18, 825-831, doi:10.1515/pjvs-2015-0107.
- Barba-Vidal, E.; Roll, V.F.B.; Castillejos, L.; Guerra-Ordaz, A.A.; Manteca, X.; Mallo, J.J.; Martin-Orue, S.M. Response to a Salmonella Typhimurium challenge in piglets supplemented with protected sodium butyrate or Bacillus licheniformis: effects on performance, intestinal health and behavior(,2). Transl Anim Sci 2017, 1, 186-200, doi:10.2527/tas2017.0021.
- Ahmed, S.T.; Mun, H.S.; Yoe, H.; Yang, C.J. Monitoring of behavior using a video-recording system for recognition of Salmonella infection in experimentally infected growing pigs. Animal 2015, 9, 115-121, doi:10.1017/S1751731114002213.
- Hurd, H.S.; Gailey, J.K.; McKean, J.D.; Rostagno, M.H. Rapid infection in market-weight swine following exposure to a Salmonella typhimurium-contaminated environment. Am J Vet Res 2001, 62, 1194-1197, doi:10.2460/ajvr.2001.62.1194.
- Zeilinger, K.; Wessels, A.G.; Vahjen, W.; Zentek, J. Effects of a pre- and probiotic mixture and an autogenous vaccine on growth performance in newly weaned piglets experimentally challenged with an enterotoxigenic Escherichia coli strain. Transl Anim Sci 2023, 7, txad030, doi:10.1093/tas/txad030.
back

